Russia claims its COVID-19 vaccine is more effective than the US, up to 92%.
- Tram Ho
Less than three days after the US pharmaceutical company Pfizer declared the COVID-19 vaccine it was developing to be more than 90 percent effective , Russian scientists said their Sputnik V vaccine was effective. up to 92% in phase III of a clinical trial.
The information has just been released by the Gamaleya Research Institute of Epidemiology and Microbiology under the Ministry of Health of the Russian Federation in Moscow after analyzing data from 16,000 volunteers participating. The volunteers were divided into two groups: one group was given 2 doses of Sputnik V three weeks apart, the other received only a placebo of saline.
All the volunteers were not told which group they belong to. After getting vaccinated, they go back home and do things like normal. During the follow-up from August until now, only 20 volunteers were infected with COVID-19.
” According to the statistical analysis of 20 confirmed corona virus infections, the division of cases between vaccinated people and those who received a placebo showed that the Sputnik V vaccine has an efficacy rate of 92% after the second dose, “ the Gamaleya Institute press release said.
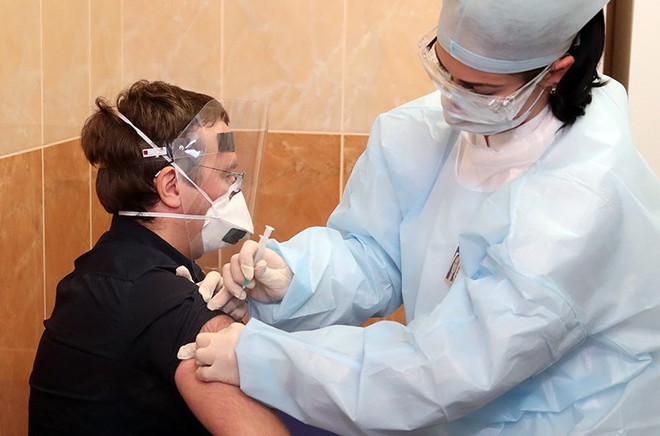
A volunteer tests Russia’s COVID-19 Sputnik V vaccine.
In addition, another trial performed on 10,000 medical workers in Russian hospitals, which are considered the front lines of the pandemic, also confirmed the effectiveness of the Sputnik V vaccine on volunteers. ” inside the red zone” is above 90%.
Russian scientists also said their vaccine is very safe. ” No unexpected side effects have been reported during this experimental period, ” the press release wrote.
The mild side effects that volunteers may experience after the Sputnik V injection are limited to the pain response at the injection site, with flu-like symptoms such as fatigue and mild headache. But those are all short-term reactions that are not worrying, the Russian researchers said.
Russia says its vaccines are superior to those from China and the West
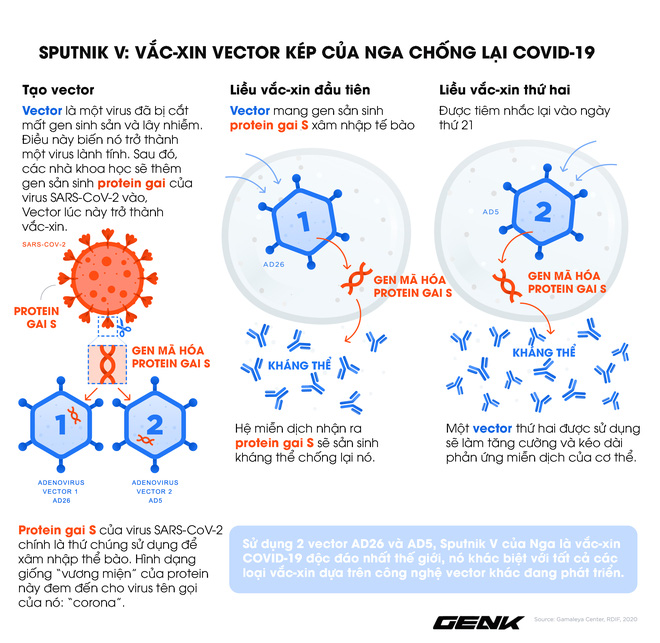
Vaccine Sputnik V was developed by the Gamaleya Research Institute in cooperation with the Russian Ministry of Defense based on virus vector technology. It is the same technology they used to develop vaccines for Ebola and MERS before.
Basically, vaccines are vector-based meaning that scientists would choose a harmless virus, in this case Adenovirus, a virus that causes the common cold. They removed its infectious gene to prevent the virus from causing disease.
Then, Adenovirus was implanted with a sequence of the SARS-CoV-2 virus that is causing the COVID-19 pandemic. These are the genes that produce the spiny protein of SARS-CoV-2 or the outer shell that it uses to attack cells.
The Adenovirus vectors now carry the thorn protein of SARS-CoV-2 that is injected into the human body. Our immune system recognizes these proteins, thereby generating antibodies against them – while the Adenovirus itself cannot make us sick.
And since the body has been trained in advance and already has an “army of antibodies” available against the spiny protein of SARS-CoV-2, when we are actually exposed to the virus, the body is now able to destroy. they and the person getting the vaccine will be immune to COVID-19.
The development direction of COVID-19 vaccine using adenovirus is being pursued by many pharmaceutical companies in many countries around the world. These include major pharmaceutical companies such as CanSino Biologics of China, AstraZeneca of England under cooperation with Oxford University, Johnson & Johnson of America in cooperation with Harvard Medical School.
Several smaller biotech companies are pursuing this vaccine development for COVID-19 including Italian ReiThera, British Stabilitech BioPharma, ImmunityBio, Altimmune Intranasal and US Vaxart.
But what all the Western vector-based vaccines have in common is that they use only a single adenovirus to carry the viral spike protein-producing gene SARS-CoV-2. Russian scientists at the Gamaleya Research Institute employ up to two.
Russian Sputnik V is essentially two different vaccines, given 21 days apart. The first dose using the vector is the AD26 virus (the same vaccine that is being developed by Johnson & Johnson and Harvard Medical School). The second dose is the Ad5 vectors (the same vaccine that is being developed by CanSino Biology in China).
Russian scientists call it a groundbreaking, unique and globally unique combination. According to them, the second injection of a second vaccination after 21 days will help the immune system strengthen its ability to recognize and fight against the SARS-CoV-2 virus. At the same time, this is expected to prolong immunity more than just one dose of a vaccine or 2 doses of the same vaccine.
It will take more time to evaluate the effectiveness of Sputnik V
At the time the Gamaleya Research Institute declared Sputnik V up to 92% effective, their Phase III clinical trial was still ongoing. As of today, November 12, about 20,000 volunteers have received either a vaccination or a placebo.
The Sputnik V Phase III clinical trial is expected to run on another 20,000 volunteers. In comparison, the US vaccine Pfizer and Germany’s BioNTech nearly completed this pilot phase with 38,955 / 43,538 participating volunteers receiving either a vaccine or a placebo.
According to the Washington Post, Pfizer found 94 volunteers infected with COVID-19 after the test. Of these, only less than 9 cases in the group had been vaccinated, the majority (more than 90%) of the remaining cases were people who received only placebo. Pfizer relies on this to claim their vaccine is more than 90% effective.
Stephen Evans, an epidemiologist at the London School of Hygiene and Tropical Medicine, said the number of COVID-19 infections reported from the Sputnik V vaccine trial stopped at just 20, much lower. American test.
The Sputnik V test protocol is currently not publicly available, in contrast to Pfizer’s protocol and some of the other top contenders in the Phase III clinical trial, so it is not clear whether an interim analysis after confirmation. determined whether 20 cases of Russian COVID-19 infection are reliable or not.
According to Evans, based on data from the Sputnik V test, he believes the Russian vaccine is only about 60% effective. “We need more monitoring because the results are compatible with much lower efficiency , “ Evans said.
Shane Crotty, vaccine immunologist at La Jolla Institute of Immunology in California agrees. He said it would be difficult to explain the results of a clinical trial without more information. ” If it were me, I wouldn’t conclude anything from 20 COVID-19 infections ,” Crotty said.
Pfizer also initially planned to perform an interim analysis after they found 32 volunteers participating in the COVID-19 infection test. However, the US Food and Drug Administration (FDA) says it needs to accumulate more cases to do so. And Pfizer waited for the number to reach 94 to declare. They plan to continue testing until they find 164 volunteers infected with COVID-19.
Eleanor Riley, an immunologist at the University of Edinburgh, UK, suspects that Russian scientists are just trying to get results against the US and other countries in the race to develop the COVID-19 vaccine.
” I am concerned that these data have been released (in a hurry) following Pfizer / BioNTech’s announcement ,” said Riley. ” But this is not a contest. We need to perform all testing to the highest standards possible.”
See Technologynetworks, Nature
Source : Genk
