Good news for the “made in Vietnam” COVID-19 vaccine: 13,000 volunteers of the 2nd phase of Nanocovax have stable health, no sudden reaction
- Tram Ho
If Nano Covax completes phase 3 trials with good results, approved by the National Ethics Council, it will be considered for emergency authorization for anti-epidemic.
At a meeting on the progress of clinical trials of a “made in Vietnam” COVID-19 vaccine on July 17, Deputy Minister of Health Tran Van Thuan said that in 2021, there is at least one COVID-19 vaccine. successfully produced domestically.
Specific information about the vaccine is expected this year, a leader of the Ministry of Health said that the Nano Covax vaccine (of Nanogen) is currently in phase 3 trials and COVIVAC (phase 2 trials).
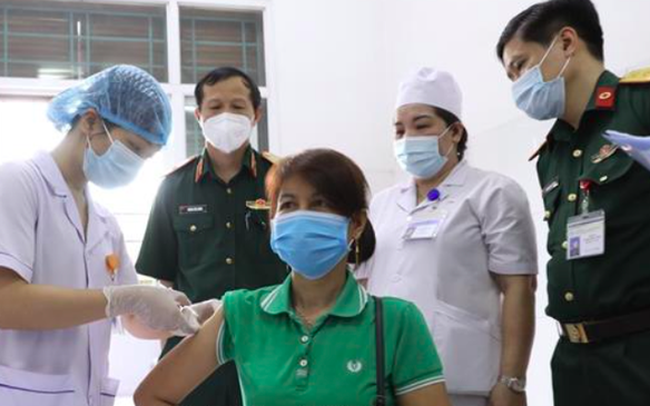
In which, from June 2021 to mid-July 2021, Nano Covax injected 1st dose to 14,000 volunteers, confirmed to be safe and evaluated for good immunogenicity, but currently there is not enough time to evaluate this effect. efficacy (the time during which the injector can be adequately protected following the injection as recommended by the manufacturer).
It is expected that, if Nano Covax completes the phase 3 TNLS with good results, approved by the National Ethics Council, it will be considered for emergency licensing for anti-epidemic.
However, this special licensing will be based on specific conditions: epidemic situation; vaccine supply is scarce, and must be submitted to the Government and the National Assembly for approval.
At the earliest, in November this year, there will be a “made in Vietnam” COVID-19 vaccine that will be widely injected. According to the manufacturer’s report, the capacity can reach 5-10 million doses/month.
Units are preparing to complete the second injection for 1,000 volunteers in the first phase, participating in the phase 3 trial of the Nano Covax vaccine.
A representative of the Military Medical Academy said that after the injection, 13,000 volunteers (phase 2) were in stable health, there were no cases of unexpected reactions. Currently, medical forces and doctors are actively calling to guide and urge volunteers to enter daily electronic monitoring log data (eDiary) related to side effects (if any) after the trial injection. experience.
Participating in the phase 3 TNLS, volunteers aged 18-75 only need to check the blood count and check for antibodies to SARS-CoV-2. People who have been exposed to SARS-CoV-2 or already have antibodies will be excluded from the test population.
It is expected that the second trial of the Nano Covax vaccine phase 3 will end before August 15.
Source : Genk
