Pfizer vaccine is safe for children 5-11 years old, testing is continuing in infants 6 months to 5 years old
- Tram Ho
Pfizer
Pfizer and BioNTech’s latest trial report update shows that their COVID-19 vaccine is safe and produces a strong immune response in children aged 5 to 11 years.
This is an important basis for Pfizer to apply for approval to health regulators, in order to get their shots early and protect children against COVID-19, especially when the variant Delta is leading to more hospitalizations and deaths in this age group.
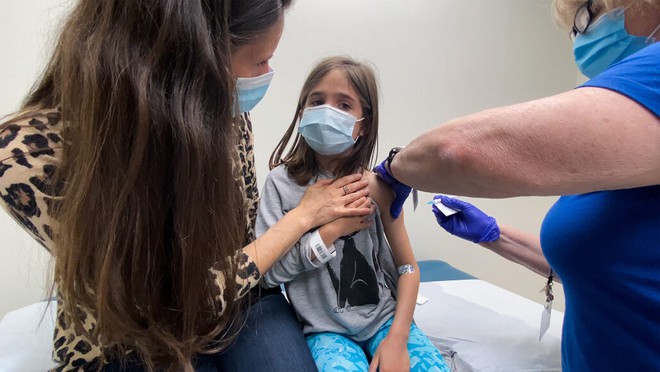
Approximately 4,500 children aged 6 months to 11 years are participating in the Phase II clinical trial of Pfizer-BioNTech’s COVID-19 vaccine.
Pfizer and BioNTech scientists say the mRNA vaccine for children aged 5-11 years has been fine-tuned to reduce the dose to a third of the vaccine for adults and children over 12 years of age. .
” In participants aged 5 to 11, the vaccine was safe, well tolerated, and showed a strong neutralizing antibody response, ” said Pfizer and BioNTech.
Ashish Jha, dean of the School of Public Health at Brown University, a leading expert on COVID-19 in the US, calls this the “good news” many parents have been waiting for. If all goes well and Pfizer’s vaccine is approved, ” my 9-year-old will get a shot this Halloween! ” he wrote in a tweet on Twitter.
In fact, Pfizer and BioNTech were the first to complete a trial of a COVID-19 vaccine for children under 12 years of age. A similar trial with Moderna’s COVID-19 vaccine is underway on children aged 6-11.
Prior to that, their adult mRNA injections were used by several countries to inject adolescents 12 years of age and older. England, Scotland and Wales also this week started Pfizer and Moderna’s COVID-19 vaccines for children aged 12 to 15. Northern Ireland will roll out similar shots next month.
Children need to be protected from the Delta variant
In the past, data has shown that children are at low risk for severe COVID-19. But the recent arrival of the Delta variant has raised concerns. The easier spread of this variant could lead to more hospitalizations in children and more severe illness.
In addition, children’s immunizations are also seen as the key to keeping schools open. It is also the final step to ending the pandemic, because as long as children are unprotected, they can still become a target for spreading disease.
“Since July, the number of COVID-19 cases in children in the US has increased by about 240%,” said Pfizer CEO Albert Bourla. ” Therefore, we are eager to extend the protection afforded by vaccines to this younger population.”
To keep schools open and end the pandemic, children also need to be vaccinated.
In the new trial, about 1,000 more children aged 5-11 received two doses of Pfizer-BioNTech vaccine, 10 micrograms each instead of 30 micrograms, compared to children over 12 years of age and adults. The injections were also shortened to just 21 days apart instead of 28 days.
The manufacturer’s representative said the 10 microgram dose had been ” carefully selected as the preferred dose for safety, tolerability and immunogenicity ” in the 5-11 age group. Side effects from the vaccine ” generally comparable to those observed in participants aged 16 to 25 ” include: pain and swelling at the injection site, headache, chills and fever.
However, the Pfizer-BioNTech press release does not address the possibility of myocarditis, a rare side effect once observed in young men receiving the mRNA vaccine.
Vaccine trials for infants and children under 5 years old
Legally, after Pfizer’s COVID-19 vaccine received full and official approval in the U.S. in August, it could have been administered to a younger population under the prescription of Pfizer. doctor.
But US authorities have warned doctors not to do this until Pfizer and BioNTech have new safety data. The US Food and Drug Administration (FDA) says it These data will be carefully considered prior to the emergency authorization of the vaccine to children under 12 years of age.
Pfizer’s COVID-19 shots for children are one-third the dose of adults.
In some countries such as Israel, the authorities seem unable to wait. Israel has specifically authorized a lower dose of Pfizer’s COVID-19 vaccine to be administered to children aged 5-11 years who are at “significant risk of serious illness or death ” if infected with COVID-19.
Meanwhile, Pfizer and BioNTech are also conducting vaccine trials on infants 6 to 24 months and children 2 to 5 years old. The results of these two trials will be available in the fourth quarter of this year at the earliest.
In total, approximately 4,500 children aged 6 months to 11 years in the US, Finland, Poland and Spain have signed up to participate in the Pfizer-BioNTech COVID-19 vaccine trial. The two companies said they will continuously update the data of the trial to the authorities in the European Union, the United States and around the world.
Refer to Sciencealert
Source : Genk
