Americans have been able to test COVID-19 at home, with the first FDA-approved kit
- Tram Ho
The latest press release from the US Food and Drug Administration (FDA) says it has licensed the first home-based COVID-19 test kit available on the market. The kit, called Pixel, is manufactured and distributed by LabCorp – a company specializing in diagnostic testing in the United States.
The Pixel consists of a Q-tip cotton swab, a sample container, a biological sealing bag and a container for transporting the sample.
LabCorp said it will sell the test box through an online registration portal. After that, the buyer will receive the goods via express mail. They will take a test sample in their nose, and then send it back to LabCorp’s lab for analysis.
Sample analysis procedures based on standard RT-PCR assays with high accuracy have also been approved by the FDA. Results will be returned online on the system, based on information that Pixel buyers have previously registered.
The price of both the kit and the testing service is 119 USD, equivalent to 2.8 million VND.

The US FDA licensed the first set of COVID-19 test kits at home
The FDA has licensed LabCorp’s COVID-19 test kit under an Emergency Use Authorization (UEA). This means that the review and approval process takes place faster than normal to respond to the pandemic emergency.
Since March, a number of biotech startups in the United States have announced the successful development of COVID-19 test kits at home. They have also tested them on their own.
The FDA said that in the race to develop the COVID-19 test, at least 350 participants and 50 tests were completed. However, the FDA emphasizes that LabCorp’s Pixel kit is now the first and only test kit they are licensed based on UEA so that people can use it safely and effectively at home.
LabCorp is a US medical diagnostic company with over 40 years of experience. Previously, they distributed home test kits for colorectal cancer, diabetes and cardiovascular disease.
It seems that the FDA is prioritizing companies with long experience in the field of home testing and sampling at home. This can help reduce errors in COVID-19 test operation, including many stages such as collection, packaging and logistics.

The home COVID-19 test kit still uses nasal solution, which is then analyzed by RT_PCR.
” During this pandemic, we made every effort to enable manufacturers to develop tests, to ensure patients have access to accurate diagnostics, including assistance in developing options. reliable and accurate home model, “said FDA Commissioner Stephen M. Hahn.
A home test kit COVID-19 can be very beneficial. First, it does not require the patient to go to a clinic or hospital, a factor that carries the risk of infection to both sides when they are having COVID-19 symptoms.
Secondly, it can free up resources as medical staff, they will not need to spend time sampling for each patient anymore. This also saves the equipment and personal protective equipment such as masks, gloves, face shields, medical protective clothing are scarce.
Third, since the RT-PCR tests on the final sample are still performed by biomedical technicians, it will still ensure the same accuracy as conventional hospital testing.
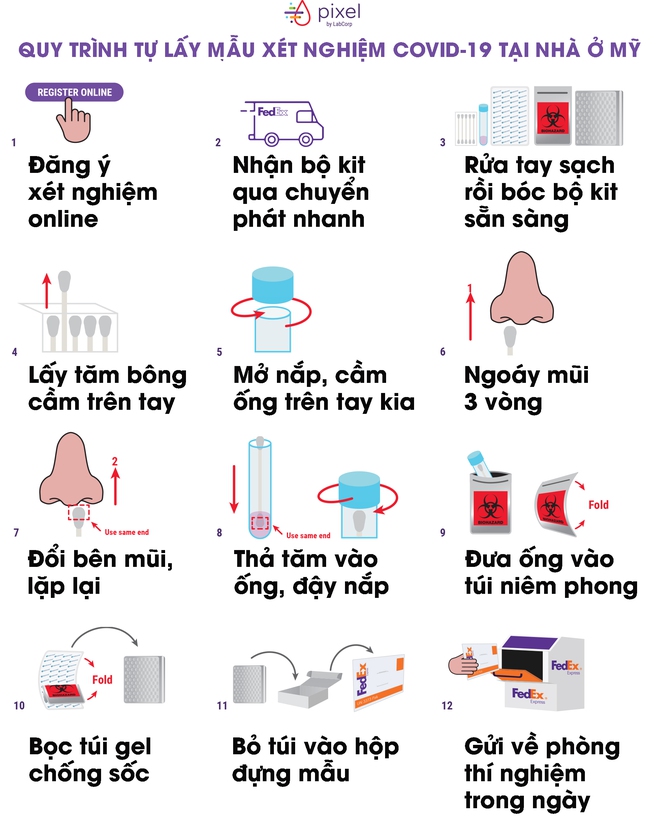
LabCorp shows how to use their Pixel kit very simply. First, after receiving the goods from the courier, place it in a dry place and wash your hands thoroughly. After that, peel off the package and take a cotton swab.
Hold the tip of this cotton swab in one hand, open the other hand and hold the sample container (be careful not to spill the water out of the tube). When you are ready, put the cotton swab on one side of the nose and swab the nose of the nose 3 times (note not to pick too deep, just enough so that the cotton tip is no longer exposed outside the nose).
Pull out the cotton swab and repeat with the other side of the nose, also spinning 3 rounds (note that one cotton tip is still used). When you’re done, you place the tip of the toothpick containing the sample in the sample container, the tip of the head dipped into the nose and dip into the liquid. Close the tube cap, place it in a bio-sealed bag.
Finally, fold the seal in half and place it on the shockproof gel bag. You fold the gel bag over the sealing bag and place it in the sample container. Return it to the courier of the day to return the sample to the lab.
The test results will be returned to you via the original online registration portal.

LabCorp said taking the COVID-19 test at home would save a lot of medical resources for the United States in this critical time.
According to data from Worldometer, the US currently has more than 800,000 COVID-19 infections, with more than 43,000 deaths. They are still performing more than 147,000 tests of COVID-19 every day at hospitals and mobile testing points.
However, experts say the United States still needs more tests to assess the extent of the pandemic. Home sampling kits like the Pixel are expected to fill that gap. LabCorp said it will deliver the first shipment to US consumers within the next few weeks.
Refer to FDA, Theverge
Source : Genk
